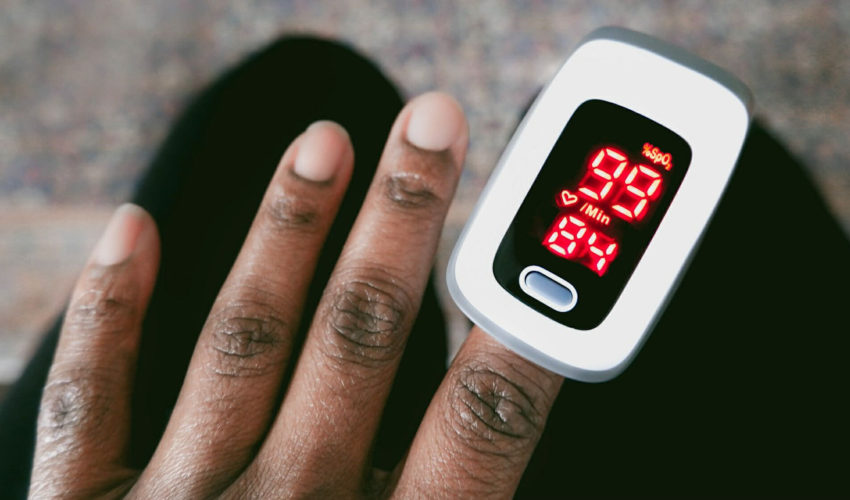
Salt Lake City – Utah Attorney General Sean D. Reyes today joined a multistate coalition of 25 attorneys general in submitting a letter to urge the U.S. Food and Drug Administration (FDA) to strengthen guidance and provide clear warning labels regarding incorrect pulse oximeter readings for patients of color. This letter comes on the heels of the first anniversary of the first public convening of the FDA’s Medical Devices Advisory Committee to address concerns about pulse oximeters’ race and color bias.
Pulse oximeters measure blood oxygen levels, which have shaped medical care for illnesses including heart failure, sleep apnea, and respiratory conditions. First invented in the 1980s and developed primarily based on tests involving a predominantly white population, pulse oximeters are now widely used throughout the healthcare system because they are the only rapid, noninvasive method of measuring oxygen saturation.
Recent studies have indicated that inaccurate results from pulse oximeters are more likely when used to monitor patients of color, particularly patients with darker skin. The medical device’s inaccuracies may lead to delays in treatment and hospital admissions, which can lead to severe or life-threatening consequences and contribute to lower quality healthcare. In the letter, the coalition calls for the FDA to issue guidance and updated warning labels regarding the risks of skin tone-based bias in pulse oximeter use.
Pulse oximeter use dramatically increased during the COVID-19 pandemic, when readings were sometimes the only objective measure for determining what kind of treatment a COVID-19 patient would get. When healthcare resources were stretched thin and subjected to rationing, a patient’s pulse oximeter reading could mean the difference between being allowed to come into the hospital for treatment versus monitoring symptoms at home or accessing lifesaving medication. Researchers have known for decades that darker skin tones could reduce pulse oximeter accuracy, but the COVID-19 pandemic led to a significant increase in evidence and broader awareness of the implications of this bias.
In the letter, the coalition commends the FDA’s implementation of safety communications about pulse oximeter accuracy and the convening of the Medical Devices Advisory Committee to address further the negative impacts of this device on patients of color. However, the FDA has yet to issue clear warning labels or provide additional guidance to protect individuals from harm as pulse oximeters are currently sold over-the-counter and used in hospitals and clinics.
In the letter, the attorneys general request that the FDA take the following steps, including:
- Requiring manufacturers and vendors of pulse oximeters to include warning labels for all users about the device’s reduced effectiveness based on skin tones.
- Calling for the inclusion of similar warnings in other medical devices that incorporate pulse oximeter readings, such as medical device software used for diagnosis or treatment of health conditions.
- Issuing guidance to healthcare providers about the risks and reduced efficacy of pulse oximeters for patients of color.
- Implementing an expedited timeline for the FDA to review the Medical Devices Advisory Committee’s recommendations.
In filing the letter, Attorney General Reyes joins the attorneys general of Arizona, California, Colorado, Connecticut, Delaware, the District of Columbia, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Jersey, New Mexico, New York, North Carolina, Ohio, Oregon, Pennsylvania, Rhode Island, Vermont, Washington, and Wisconsin.